Abstract
Background CD19-directed chimeric antigen receptor T-cell (CAR-T) products are now part of standard of care (SOC) treatment for advanced large B cell lymphoma (LBCL). Because of concerns of potential immune cell-associated neurotoxicity syndrome (ICANS), patients with secondary central nervous system (CNS) manifestation of lymphoma have been excluded from most pivotal CAR-T studies. Consequently, data on outcome of CAR-T cell therapy for lymphoma patients with secondary CNS involvement are limited. Here, we show results of CD19 CAR-T cell therapy for relapsed/refractory secondary CNS lymphoma in a German multicenter real-world cohort.
Patients and Methods 196 LBCL patients from the EBMT/DRST registry who had received SOC CD19 CAR-T cell therapy in 9 German centers between 2018 and 2021with sufficient detailed information on type of CNS involvementat the time of CAR-T therapy were included. In total, 28 patients with and 168 patients without secondary CNS involvement were identified. For the purpose of a more representative comparison, a matched-pair analysis of the 2 groups was performed using age, sex, performance status, and International Prognostic Index as matching factors.
Results Of the 28 patients with CNS involvement, in 8 patients CNS was involved at initial presentation of lymphoma. Regarding the type of CNS manifestations, 16 patients (57%) had lymphoma manifestations in the brain only, 6 patients (21%) had meningeosis only and remaining 6 patients (21%) had both.
14 patients (50%) received axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) respectively after a median of 3 lines of prior treatments (range, 2-6 lines). 24 patients (86%) received bridging therapy before CAR-T infusion. Bridging included intrathecal therapy in 4 patients (14%), polatuzumab-based regimen in 5 patients (18%), CNS radiation in 3 patients (11%), venetoclax/ibrutinib in 4 patients (14%), gemcitabine-oxaliplatin in 2 patients (7%), other chemotherapies in 6 patients (25%).
Median age was 58 years (range, 33-79 years) and median time from diagnosis to CAR-T infusion was 15 months (range, 4-105 months). 16 patients (57%) were male, with a median ECOG performance status of 1 (range, 0-3) and median International Prognostic Index of 3 (range, 1-5). Thirteen patients (48%) had normal levels of LDH at time of CAR-T. Median time to last documented follow-up of all patients (alive or dead) was 10 months (range 0.3-33 months).
In terms of safety, CRS of any grade was seen in 24 patients (86%), grade 3 in 6 patients (21%). There was no grade 4 event. ICANS occurred in 13 patients (46%), grade 3 in 3 patients (11%), and grade 4 in 1 patient (4%). Median time to ICANS was 5 days (range, 3-15 days) after CAR-T infusion. No difference in occurrence of and time to ICANS was seen between axi-cel and tisa-cel (P=0.81 and 0.76) or type of CNS manifestation (P=0.15 and 0.52).
In terms of efficacy, complete response rate was 33%, partial response rate 33%, progressive disease 25%, and stable disease 9%. Median progression-free survival of the total CNS cohort was 3.8 months (95% CI, 0.01-9.4 months). Progression-free survival (PFS) was significantly affected by type of manifestation (P=0.006), showing median of 3.8 months (95% CI, 0.01-11.9 months) for brain only manifestation versus 1.1 months (95% CI, 0.01-2.6 months) for meningeosis only versus not reached for others (both meningeosis and brain). 3 events of non-relapse mortality occurred, all due to blood stream infections at day 10, 13, and 27 after CAR-T infusion.
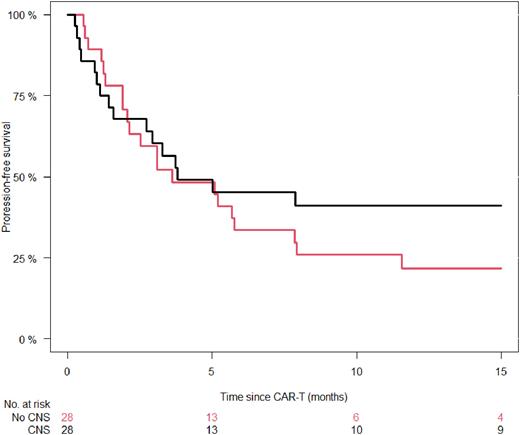
In a matched comparison of 28 matched patients with and without CNS involvement, no difference was seen for incidence of CRS (P=0.43) and ICANS (P=0.71) between the groups. PFS was comparable (P=0.26), showing a median of 3.6 months (95% CI 0.4-6.9 months) for no CNS involvement versus 3.8 months for CNS involvement. PFS at 6 months was 45% (26-64%) versus 34% (16-52%) and at 12 months 41% (22-60%) versus 22% (6-38%) for patients with CNS vs. no CNS manifestation (Fig 1).
Conclusion SOC CD19 CAR-T cell therapy for LBCL patients with secondary CNS involvement is safe, feasible, and results in efficacy comparable to outcome in those without CNS involvement These findings not only underline that CNS involvement should not preclude patients from clinical trials, but also the high potential of cell therapies for CNS lymphomas.
Disclosures
Ayuk:Medac: Honoraria; Novartis: Honoraria; Celgene/BMS: Honoraria; Takeda: Honoraria; Mallinckrodt/Therakos: Honoraria, Research Funding; Miltenyi Biomedicine: Honoraria; Janssen: Honoraria; Gilead: Honoraria. Wulf:Gilead: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. von Tresckow:Abbvie: Other: NA; Allogene: Consultancy; Amgen: Consultancy; AstraZeneca: Honoraria, Other: NA; BMS: Honoraria, Other: NA; Cerus: Consultancy; Gilead/Kite: Consultancy, Honoraria, Other: NA, Research Funding; Incyte: Consultancy, Honoraria; IQVIA: Consultancy; Miltenyi: Consultancy; MSD: Consultancy, Honoraria, Other: NA, Research Funding; Novartis: Consultancy, Honoraria, Other: NA, Research Funding; Pentixafarm: Consultancy; Pfizer: Consultancy; Roche: Consultancy, Honoraria, Other: NA; Takeda: Consultancy, Honoraria, Other: NA, Research Funding. Rejeski:Kite/Gilead: Other: Travel Support, Research Funding; Novartis: Honoraria. Stelljes:Kite: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Amgen: Consultancy; MSD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Medac: Honoraria. Penack:Shionogi: Membership on an entity's Board of Directors or advisory committees; SOBI: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Priothera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Omeros: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Equillium Bio: Membership on an entity's Board of Directors or advisory committees; Therakos: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Dreger:AbbVie, AstraZeneca, Gilead, Novartis, Riemser, and Roche: Speakers Bureau; AbbVie, AstraZeneca, Bluebird Bio, Gilead, Janssen, Novartis, Riemser, and Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal